Laws of Thermodynamics
- Get link
- Other Apps
Laws of Thermodynamics: Understanding the Principles that Govern Energy and Heat
Introduction:
The laws of thermodynamics are fundamental principles that govern the behavior of energy and heat in physical systems. They provide a framework for understanding the transfer, conversion, and utilization of energy in various processes. Developed through years of scientific inquiry, the laws of thermodynamics form the cornerstone of modern physics, engineering, and many other scientific disciplines. In this article, we will explore the four laws of thermodynamics, their significance, and their applications in different fields.
1. Zeroth Law of Thermodynamics:
The zeroth law of thermodynamics establishes the concept of thermal equilibrium and the measurement of temperature. It states that if two systems are separately in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. This law allows for the definition of a temperature scale and enables the comparison of the thermal states of different systems.
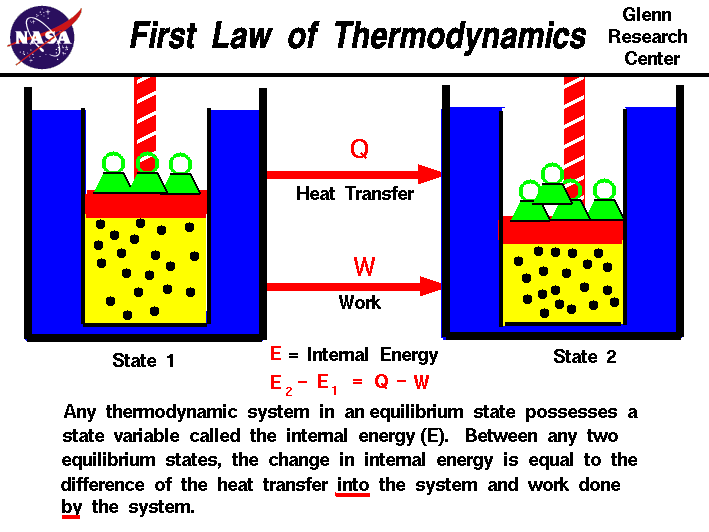
2. First Law of Thermodynamics (Law of Energy Conservation):
The first law of thermodynamics, also known as the law of energy conservation, states that energy cannot be created or destroyed in an isolated system. It can only be converted from one form to another or transferred between different components of the system. The first law establishes the principle of energy conservation, which is fundamental in understanding energy transformations in various processes, including mechanical work, heat transfer, and chemical reactions.
3. Second Law of Thermodynamics:
The second law of thermodynamics introduces the concept of entropy and the directionality of natural processes. It states that the entropy of an isolated system tends to increase over time. Entropy can be thought of as a measure of the disorder or randomness in a system. The second law also establishes the concept of heat flow from a higher temperature region to a lower temperature region, which defines the direction of spontaneous heat transfer.
4. Third Law of Thermodynamics:
The third law of thermodynamics states that as the temperature of a system approaches absolute zero (0 Kelvin or -273.15 degrees Celsius), the entropy of the system approaches a minimum or a constant value. It implies that at absolute zero, a perfect crystal would have zero entropy. The third law provides a basis for understanding the behavior of materials at extremely low temperatures and their relationship to entropy and energy.
Applications of the Laws of Thermodynamics:
The laws of thermodynamics have wide-ranging applications in various scientific and engineering fields. Some notable applications include:
1. Energy Conversion and Efficiency:
The laws of thermodynamics are crucial in designing and optimizing energy conversion systems such as heat engines, power plants, and renewable energy technologies. They help determine the efficiency and performance of these systems by analyzing the energy input, output, and losses during energy transformations.
2. Chemical Reactions and Kinetics:
Thermodynamics plays a significant role in chemical reactions and understanding reaction spontaneity, equilibrium, and the direction of reaction. It helps predict the feasibility and efficiency of chemical processes and guides the design of chemical reactors.
3. Heat Transfer and Thermal Engineering:
Thermodynamics provides the foundation for studying heat transfer mechanisms such as conduction, convection, and radiation. It enables engineers to design efficient heat exchangers, HVAC systems, and thermal management solutions in various industries.
4. Refrigeration and Cryogenics:
Thermodynamics is essential in the design and operation of refrigeration and cryogenic systems. It enables the analysis of cooling processes, heat pumps, and the behavior of materials at extremely low temperatures.
5. Material Science and Phase Transitions:
The laws of thermodynamics are crucial in understanding phase transitions, such as melting, freezing, and vaporization. They guide the study of material properties, phase diagrams, and the behavior of substances under different conditions.
Conclusion:
The laws of thermodynamics form the basis for understanding energy, heat, and their interactions in physical systems.
They provide essential principles for engineers, scientists, and researchers working in various fields, from energy conversion to material science. By applying these laws, we can gain insights into the behavior of systems, predict the direction of natural processes, and optimize energy utilization. The laws of thermodynamics are not only fundamental to our understanding of the physical world but also play a crucial role in shaping technological advancements and sustainable practices.
- Get link
- Other Apps
Comments
Post a Comment